- Health system policies to ensure the integration of palliative care into the structure and financing of national health care systems at all levels of care;
- Policies for strengthening and expanding human resources, including education and training of health care professionals, in order to ensure adequate responses to the palliative care needs, together with training of volunteers and education of the public;
- A medicines policy in order to ensure the availability of essential medicines for the management of symptoms, including pain and psychological distress, and in particular, opioid analgesics for relief of pain and respiratory distress;
- A policy for research into assessing the needs for palliative care and identifying standards and models of service that work, particularly in limited resource settings.http://apps.who.int/gb/ebwha/pdf_files/EB134/B134_28-en.pdf. '[73]
A World Health Assembly resolution on palliative care adopted on May 23, 2014, which México co-sponsored, closely mirrors these recommendations.[74]
These recommendations also correspond closely with several obligations under the right to health. The Committee on Economic, Social and Cultural Rights, the body that monitors the implementation of the right to health as articulated in the International Covenant on Economic, Social and Cultural Rights (ICESCR),[75] has held that countries must adopt and implement a national public health strategy and plan of action and ensure access to essential medicines as defined by the WHO.[76] It has identified providing appropriate training for health personnel as an obligation “of comparable priority.”[77] Failure to take steps in these three areas may result in a violation of the right to health.
Integration of Palliative Care into the Healthcare System
According to the WHO, national health system policies should promote the integration of palliative care into the structure and financing of national health care systems at all levels of care. In these policies, the emphasis should be on primary care, community and home-based care.[78] The WHO also recommends that palliative care be part of efforts to promote universal health coverage.
The right to health requires states to take the steps necessary for the “creation of conditions which would assure to all medical service and medical attention in the event of sickness” (emphasis added).[79] The Committee on Economic, Social and Cultural Rights has held that people are entitled to a “system of health protection which provides equality of opportunity for people to enjoy the highest attainable level of health.”[80] In other words, health services should be available for all health conditions, including chronic or terminal illness, on an equitable basis.
The 2009 palliative care amendments to México’s health law are closely in line with these WHO recommendations and human rights obligations. The amendments explicitly oblige all healthcare institutions to offer palliative care services, including counseling about illness, treatment of pain and psychological support. This is only possible with the integration of palliative care into the structure and financing of the healthcare system. A February 2013 meeting on the role of palliative care in México’s national cancer strategy clearly identified this as a priority.[81]
As noted, however, implementation has lagged as the ministry of health has yet to adopt the implementing norm for the amendments. As a result the actual integration of palliative care into the structure of the healthcare system at all levels of care has not happened to date. México has made some significant advances at the tertiary level of care where a growing number of hospitals are now offering palliative care as a core part of their health services. But at secondary and primary care levels palliative care is still mostly non-existent.
Integration of Palliative Care into Health Insurance Schemes
With most of its population covered by a health insurance scheme, México has the infrastructure to deliver palliative care to most people who need it. However, the availability of palliative care services among providers in the networks of these insurers is very limited and one major health insurer only covers palliative care partially for most patients.
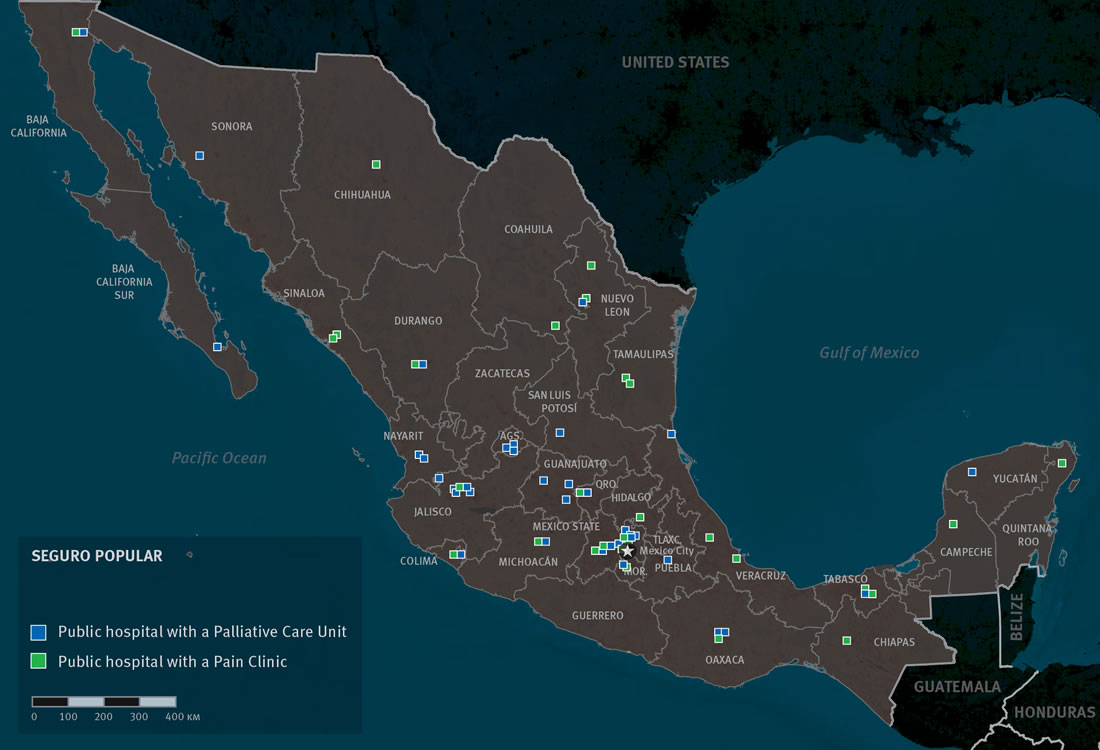
Seguro Popular
Prior to 2014, Seguro Popular did not cover palliative care for many conditions, including many cancers. The 2012 list of basic medical interventions and medicines, the Universal Catalogue of Health Services, that are covered under the insurance, for example, did not include a specific intervention for palliative care or include a number of key symptoms that many palliative care patients face, such as pain and breathlessness.[82] This meant that the several dozen public hospitals that attend to Seguro Popular patients and that do have palliative care available had to charge them for such services or had to provide them free-of-charge without possibility of being reimbursed.
To address this gap in coverage, the National Commission for Social Protection of Health (NCSPH), which administers Seguro Popular, added an intervention entitled “Attention for certain signs, symptoms, and other factors that influence the state of health” to the 2014 catalogue which includes various common symptoms in palliative care patients, including pain and breathlessness.[83] Each intervention in the catalogue comes with a list of recommended medicines. Yet, curiously, the list of medicines for the intervention on control of symptoms does not include any opioid pain medicines, even though injectable morphine, tramadol, and buprenorphine were added to Seguro Popular’s general medicines list.[84] While physicians can prescribe any of the medicines on Seguro Popular’s list for any covered condition, the lack of inclusion of key pain medicines with the intervention on symptom control may well discourage physicians from using them. The NCSPH is currently reviewing the financial feasibility of adding coverage of a more comprehensive set of palliative care interventions in the future.[85]
The Fund for the Protection against Catastrophic Costs (Fondo de Protección contra Gastos Catastróficos), which covers certain complex medical conditions that generally involve major expenditure for treatment, including all pediatric cancers, some adult cancers and HIV, also does not cover palliative care adequately.[86] While the Fund covers diagnostic tests and curative treatment for these illnesses, the Fund’s documentation is not explicit on whether its beneficiaries are entitled to receive palliative care.[87] The Fund’s essential medicines list does include various strong opioid analgesics.[88]
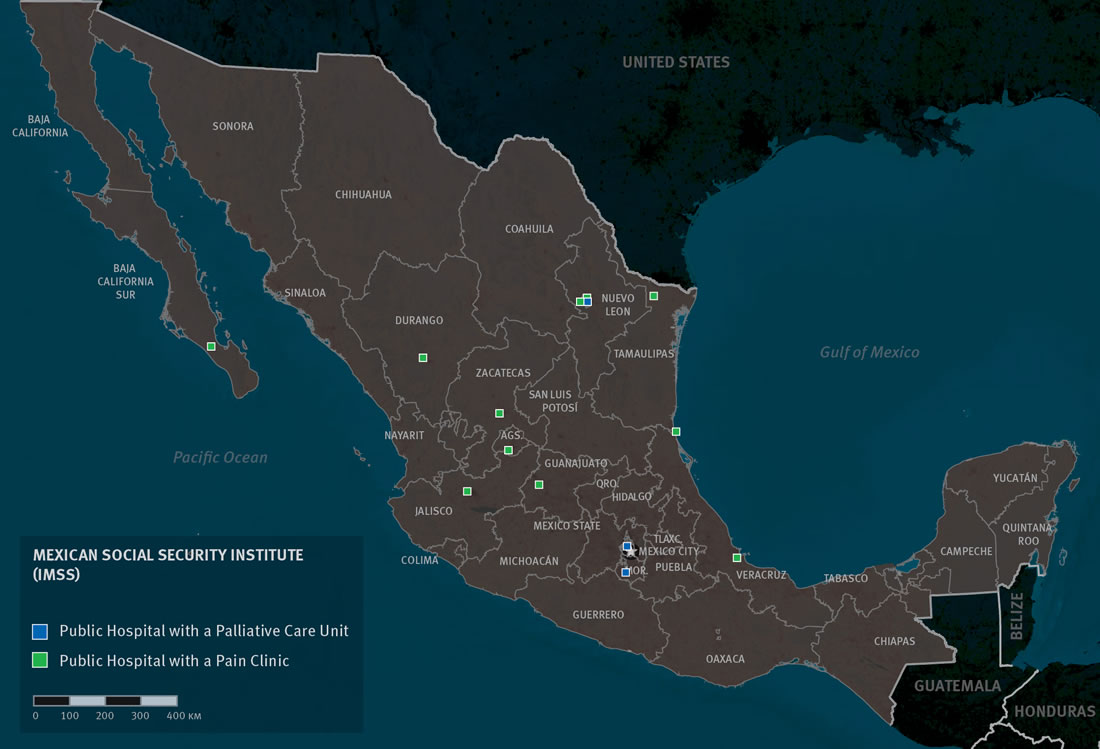
Mexican Social Security Institute (IMSS)
IMSS will cover palliative care interventions and medicines.[89] However, few IMSS hospitals and clinics actually offer palliative care services. We identified just three tertiary care hospitals within the IMSS system—in México City, Monterrey, and Cuernavaca—that offer palliative care and none at the secondary level.[90] Thus, although palliative care in theory is covered for patients with IMSS, most cannot actually access it because of its limited availability. IMSS’ medicines list includes most essential palliative care medicines that are available in México (see below under Medicines Availability), although not all are available at primary and secondary levels of care.
IMSS has a home-based care program for people with chronic illnesses at 140 of its general and specialist hospitals, which attended to 35 thousand patients in 2013 and could be a good vehicle for delivering palliative care.[91] However, at present the program is designed for patients with long-term care needs rather than for those with a prognosis of short-term survival and health needs that require immediate assistance. The program has limited capacity which means that patients with advanced illness cannot always enroll immediately. Furthermore, staff of these home-based care programs are not currently trained in the provision of palliative care.
IMSS boasts an award winning palliative care service in Monterrey, Nuevo León, that serves several states in northeastern México.[92] This service, which operates out of Unidad Medica de Alta Especialidad 25 (UMAE 25), uses a decentralized model to assist patients. The service has invested extensively in training primary care physicians in the northeastern region and refers patients to primary care givers for day-to-day care. For prescriptions for opioid analgesics and more complex symptom management patients do still need to travel to Monterrey but the cooperation with primary care givers has reduced the need for patients to travel back-and-forth. The director of UMAE 25 credits the palliative care service with a significant reduction of bed occupancy in the hospital’s oncology unit.[93]
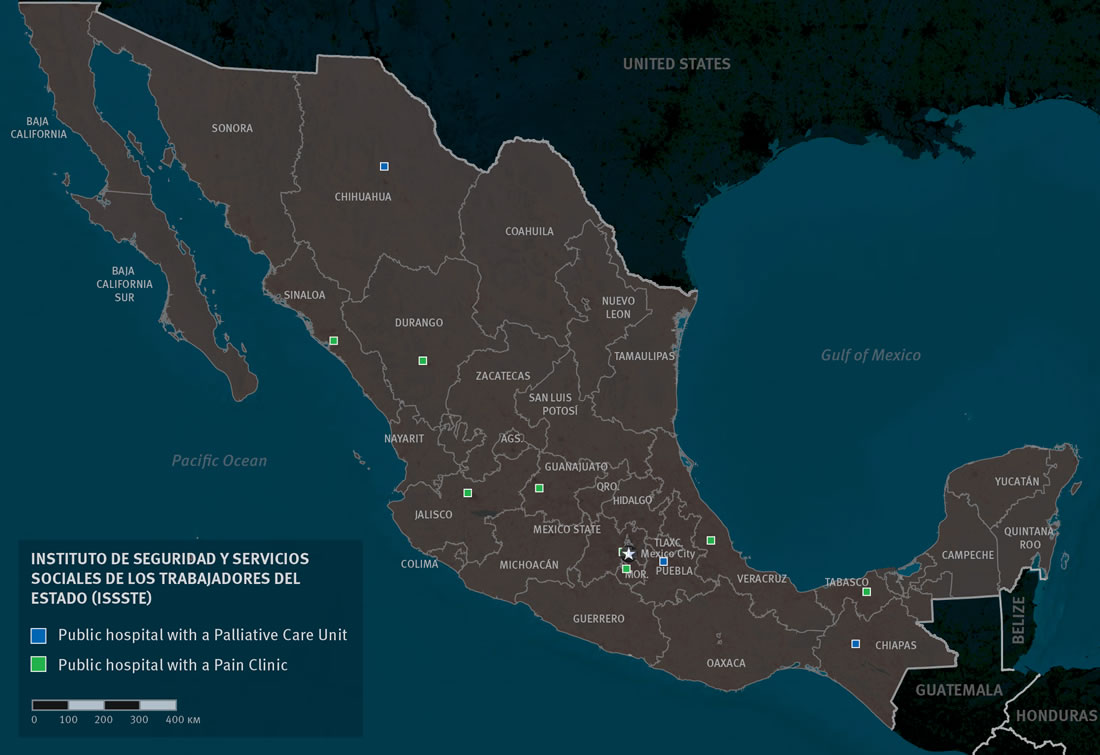
Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE)
ISSSTE does not appear to have a public list of the medical conditions and interventions it covers or a public medicines list. However, key informant interviews suggest that palliative care interventions, such as management of physical and psychosocial symptoms, are covered when available. We identified five of a total of 110 ISSSTE hospitals—in Chiapas, Chihuahua, México City, Guanajuato and Puebla—that have palliative care services.[94]
Lack of Referral Systems
The WHO and the WHA resolution recommend that people who require palliative care can receive it in their home or community. International human rights standards hold that health services should be within “safe physical reach for all sections of the population, especially vulnerable and marginalized groups.”[95] This is only possible with the existence of palliative care capacity at the primary care level, which is sorely lacking in México. It also requires a functioning referral system so that patients who become incurable are referred back to healthcare providers in or close to their communities.
As testimony from people with life-limiting illnesses and their families above shows clearly, such a system does not currently exist in México even when community or home-based palliative care services are available. Palliative care services and their capacities are currently not recorded or circulated in any way, complicating referrals. For example, organograms of México’s hospitals do not include palliative care services or pain clinics.
Some hospitals have set up their own referral systems. For example, through its extensive training activities, the palliative care unit of Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in México City has built up a network of trained physicians in many parts of the country that it refers patients to when they finish their hospital treatment so that they no longer have to travel to México City to receive palliative care services.[96] The National Cancer Institute works with a network of cancer centers that includes 33 hospitals around the country, 18 of which have palliative care units, although three of the states where most of their patients come from, Hidalgo, Morelos, and Tlaxcala, do not have regional cancer centers.[97] UMAE 25 of IMSS in Monterrey has developed its own referral system to primary physicians in Nuevo León and adjacent states by investing in training of primary care physicians in palliative care. When patients are ready to be sent home, the hospital draws up referral instructions for primary physicians so that they can offer services to patients close to their homes.[98]
Palliative Care Education for Healthcare Workers
Adequate training and education for healthcare workers in palliative care is essential to the rollout of this health service. As noted above, the Committee on Economic, Social and Cultural Rights considers appropriate training of healthcare workers an obligation of key importance.[99] Accordingly, the WHO recommends that “education about palliative care (including ethical aspects) is offered to students in undergraduate medical and nursing schools and to health care providers at all levels…”[100] The May 2014 World Health Assembly resolution calls on countries to include palliative care as an “integral component of the ongoing education and training offered to care providers” and specifies:
(a) basic training and continuing education on palliative care should be integrated as a routine element of all undergraduate medical and nursing professional education, and as part of in-service training of caregivers at the primary care level, including health care workers, caregivers addressing patients’ spiritual needs and social workers;
(b) intermediate training should be offered to all health care workers who routinely work with patients with life-threatening illnesses, including those working in oncology, infectious diseases, paediatrics, geriatrics and internal medicine;
(c) specialist palliative care training should be available to prepare health care professionals who will manage integrated care for patients with more than routine symptom management needs.[101]
To date, palliative care has not yet been integrated into curricula and continuing education programs for healthcare workers. México City and Guadalajara each have multiple training programs for different kinds of healthcare workers and at different levels but in the rest of the country such programs are almost entirely non-existent. As a result, most healthcare workers in México have no knowledge of palliative care and lack any clinical exposure to this health service, greatly complicating efforts to integrate palliative care into the healthcare system.
Research by the Latin American Palliative Care Association (ALCP) on palliative care in México demonstrates the extent of the training capacity gap (see Table 2).[102] It found that just six medical schools in México teach palliative care as part of the curriculum and that it is mandatory in just two.[103] México has a total of 102 medical schools.[104] A recent publication in the journal Palliative Medicine found that México ranked 12th of 19 countries in the region in integration of palliative care into undergraduate medical curricula.[105] According to the report, the Asociación Mexicana de Facultades y Escuelas de Medicina, which is made up of 54 medical schools and makes non-binding recommendations about medical curricula, plans to include palliative care into its model curricula.[106] México’s universities enjoy wide discretion in setting curricula for their academic programs, complicating any potential efforts by the government to ensure palliative care is taught in medical schools and other relevant university courses.
According to the ALCP study, just four nursing schools, three faculties of psychology, and two faculties of social work include palliative care as part of their curricula. The report identified forty-five medical doctors who teach palliative care and 25 non-medical palliative care trainers. It said about 250 medical doctors had completed palliative care courses.
The ALCP study did not seek to identify to what extent palliative care is integrated into continuing education programs for medical doctors, nursing staff, psychologists, pharmacists and social workers. The need for such programs is enormous as most existing healthcare workers have not had any training or clinical exposure to palliative care.
To date, there have not been any concerted efforts by the government, the healthcare system or academic institutions to systematically incorporate palliative care into training curricula and programs for healthcare workers. This may change now that the health ministry has hired a palliative care physician with extensive training experience to help it develop its work plan in this area.
Table 2
|
|
Medical schools |
Nursing schools |
Psychology |
Social work |
|
Undergraduate |
- Universidad Autónoma de Guadalajara (mandatory) - Universidad de Guadalajara (mandatory) - Universidad Panamericana (elective) - Universidad Nacional Autónoma de México (UNAM) (elective) - Universidad La Salle (elective) - Instituto Politécnico Nacional (elective) |
- Universidad de Guadalajara (mandatory).*
|
- Universidad Autónoma de Guadalajara (elective) - Universidad Jesuita de Guadalajara (ITESO) (mandatory)*
|
- UNIVA (elective)* |
|
Postgraduate (6-12 month diploma courses) |
- UNAM - Universidad de Guadalajara - Universidad Autónoma de Guadalajara |
- UNAM - Universidad de Guadalajara - Universidad Autónoma de Guadalajara - Centro de Cuidados Paliativos en México |
- UNAM - Universidad de Guadalajara - Universidad Autónoma de Guadalajara |
- UNAM - Universidad de Guadalajara - Universidad Autónoma de Guadalajara - Centro de Cuidados Paliativos en México |
|
Specialist (only for physicians) |
- Universidad Nacional Autónoma de México has a one-year specialization course for doctors specialized in internal medicine, psychiatry, geriatrics, oncology or algiology. - The National Institute of Pediatrics offers a super specialization course in pediatric palliative care for pediatricians. - The Institute Jalisciense de Alivio al Dolor y Cuidados Paliativos, the General Hospital of the West and the University of Guadalajara offer a two-year subspecialty program in palliative and pain medicine. - The Autonomous University of Guadalajara plans to start a master’s program in palliative care in March 2015. |
|||
|
* The Atlas does not list the names of the nursing schools, faculties of psychology, and faculties of social work that offer palliative care courses in their undergraduate curricula. Human Rights Watch has listed the schools and faculties it was able to confirm. |
||||
In July 2014, the Mexican Social Security Institute (IMSS), one of México’s major health insurers, decided to include children’s palliative care into the training program for residents specializing in pediatrics at its affiliate hospitals. Palliative care had previously been included in the curriculum in Guadalajara, where 8 hours of pediatric palliative care have been taught to third year residents in pediatrics since 2011 under “Emerging illnesses and problems.”[108]
Medicines Availability
The WHO recommends that countries adopt a “medicines policy in order to ensure the availability of essential medicines for the management of symptoms, including pain and psychological distress, and in particular, opioid analgesics for relief of pain and respiratory distress.”[109] In 2013, the WHO created sections on pain and palliative care in its Model List of Essential Medicines and its Model List of Essential Medicines for Children. These sections contain medicines and specification for formulations that the WHO considers essential for pain management and palliative care.
Under the right to health, countries are required to ensure the availability and accessibility of all medicines included in the WHO Model List of Essential Medicines. The Committee on Economic, Social and Cultural Rights has held that providing essential medicines as determined by the WHO is a core obligation that cannot be limited by claims of limited resources, but states should fulfill immediately.[110]
As discussed above, there are still significant challenges in México with the availability and accessibility of opioid analgesics. As a study by the Pain and Policy Studies Group at the University of Wisconsin found in 2013, Mexican law contains various provisions that strongly support the need to make opioid analgesics available for medical purposes.[111] However, implementing regulations create numerous complications in practice, which are explored in this chapter. While the situation with accessibility of other essential palliative care medicines is better, we also explore challenges in this area.
Access to Opioid Analgesics
You have to help the patient first. If we aren’t able to help the patient, the regulations are not good. - Dr. Juan José Lastra, Ajijic, Jalisco[112]
Opioid analgesics are essential for the management of pain, shortness of breath and several other common symptoms among people who need palliative care. Because opioid medicines are controlled substances, countries must regulate how they can be produced, distributed, prescribed and dispensed. The 1961 Single Convention on Narcotic Drugs, the international agreement that provides the framework for national drug control efforts, contains four basic requirements for national regulations of controlled medicines:
- Individuals dispensing the medication must be licensed, either by virtue of their professional license or through a special licensing procedure;
- The medications may only be transferred between authorized institutions or persons;
- The medications can only be dispensed to a patient upon a medical prescription;[113] and
- Records on the movement of these medications are kept for no less than two years.[114]
The Single Convention specifically allows countries to put in place additional requirements, including a special prescription form for controlled medications. Countries, however, have a dual obligation with respects to these medicines: they must ensure their adequate availability for medical and scientific use while preventing their misuse and diversion.[115] Countries must take care that any additional requirements to those specified in the 1961 Single Convention do not unnecessarily impede medical access.[116]
As noted, international human rights standards require that countries ensure the availability and accessibility of opioid analgesics that are included in the WHO Model List of Essential Medicines. As the manufacturing, prescribing, and dispensing of controlled medicines is subject to strict regulation by governments, states have an obligation to ensure these regulations do not unnecessarily restrict patient access to them for medical purposes. Any regulations that arbitrarily impede the procurement and dispensing of these medications for medical purposes are incompatible with the right to health.
Numerous challenges currently exist with the prescribing of opioid analgesics due to burdensome requirements for physicians and strict and inflexible dispensing rules for these medicines. México’s health law and a regulation on medical products and instructions from the country’s pharmaceuticals commission set out the rules for prescribing and dispensing medicines.[117]
The Mexican government, however, has announced significant changes to the prescribing system for opioid analgesics.[118] In making the announcement, COFEPRIS stated specifically that these changes aim to improve access to controlled medicines such as morphine for patients with terminal illness.[119] Under the new system, which is expected to be operational in the first quarter of 2015, many of the barriers identified here should seize to exist. The specific changes that are being prepared are discussed below, along with descriptions of current barriers.
Obtaining Prescription Rights
Under current Mexican law, a physician must obtain a special license, triplicate prescription forms, and barcoded stickers in order to be able to prescribe opioid analgesics.[120] These requirements go well beyond what the 1961 Single Convention mandates. The WHO has noted that requirements for duplicate prescriptions and special prescription forms increases the administrative burden both for healthcare workers and drug control authorities. It has stated that this problem is “compounded if forms are not readily available, or if health professionals need to pay for them.” It recommends that countries should “ensure that this system does not impede the availability and accessibility of controlled medicines.”[121]
Obtaining the license to prescribe opioid analgesics. In order to obtain the license to be allowed to prescribe opioids, the medical doctor must apply to the health department or COFEPRIS office in their state.[122] This can only be done in the state capital and must be done in person, a disincentive for doctors who work outside of capital cities. The license is generally not issued the same day, meaning doctors may have to return a second time to finalize proceedings. Although an explanatory note of the health ministry and COFEPRIS clearly states the process is free of charge, some Mexican states charge an application fee for obtaining the license, creating an additional financial burden (see Table 3 below).[123] Under the changes that are being prepared, physicians will be able to request and obtain these licenses through an online procedure and will no longer have to make physical appearances as part of this process.[124]
The special prescription form. Physicians must order special triplicate prescription forms in order to prescribe opioid analgesics, again requiring additional expenditure on their part. Problematically, the rules for these forms require that they contain the physician’s home address.[125] This requirement does not appear to serve any useful purpose from a drug control or medical perspective but does pose a potential security risk for the prescribing physician. Several doctors told us that this requirement is a deterrent. As one doctor commented, “I only give my home address to people I trust, not to everybody. So I don’t know why your house phone and your address have to be in the prescription.”[126] The government has announced that this requirement will be eliminated.[127]
Bar coded stickers. The most significant disincentive to prescribing opioid analgesics is the very unusual requirement in México’s general health law that prescriptions for these medications must carry a barcoded sticker.[128] A variety of factors makes the current system for barcoded stickers very user-unfriendly and discourages physicians from prescribing these medications:
- The stickers can only be issued by the health and sanitation authorities at one location in each state’s capitals, usually the health ministry or COFEPRIS office, which are open only during business hours.[129]
- Doctors must collect the stickers in person.[130]
- Regulations allow health departments to issue only fifty pairs of stickers at once.[131]
- In most states, the stickers are not prepared immediately so the physician must return to collect them later, sometimes several weeks later.
- Some states charge physicians for barcoded stickers (see Table 3).
- Some of the machines used to print the stickers are outdated and are reported to break down frequently, resulting in states being unable to issue new stickers for weeks or even months at a time.[132]
This system is difficult for all physicians but especially for those who live outside of state capitals. They must travel to the state capital—a trip that can often take hours—at least once for every batch of barcoded stickers they need. If the health department or COFEPRIS do not issue the stickers immediately, they may have to travel a second time. If the machine that prints the stickers is broken, a trip to the capital can be altogether futile.
A general practitioner in Ajijic, about an hour away from Guadalajara, told Human Rights Watch that he needs a new set of fifty barcoded stickers every two weeks and thus has to make the trip to the city every fortnight. He said that he loses half a day of work each time.[133] Another doctor from the same town said he has to close his private clinic early on days he needs to travel to Guadalajara to pick up barcoded stickers:
My consultations are in the mornings and they [the health department] are open only in the mornings. So some days I’ll have to go at 1pm as they close at 3pm. For me it means losing patients or seeing fewer patients.[134]
Table 3 – Fees for Licenses to Prescribe Opioids and Barcoded stickers[135]
|
State |
Charge for License |
Charge per set of 50 stickers |
Processing of Stickers |
|
|
Baja California Sur |
606 pesos (US$46.69) |
472 pesos (US$36.36) |
5 days |
|
|
Chiapas |
650 pesos (US$50.8) |
No charge |
10 days |
|
|
Colima |
No charge |
887 pesos (US$68.34) |
5 days |
|
|
México State |
318.85 pesos (US$25.56) or 336 pesos (US$25.89)* |
318.85 pesos (US$25.56) or 336 pesos (US$25.89)* |
2 days |
|
|
Guerrero |
1228 pesos (US$86.90) or 1295 pesos (US$99.77)* |
614 pesos (US$47.30) or 648 pesos (US$49.92)* |
10 days |
|
|
Querétaro |
No charge |
200 pesos (US$15.41) |
Not available |
|
|
San Luis Potosí |
638 pesos (US$49.15) |
638 pesos (US$49.15) |
Not available |
|
|
Sinaloa |
No charge |
150 pesos (US$11.56) |
Not available |
|
|
Tamaulipas |
63.77 or 67.29 pesos (US$4.91 or 5.18)]* |
63.77 or 67.29 pesos (US$4.91 or 5.18)]* |
26 days |
|
|
Yucatán |
No charge |
689 pesos (US$53.08) |
3-4 days |
* The higher fees generally apply in major urban areas.
Even for physicians who live in state capitals, collecting the barcoded stickers can be a time-consuming affair. In México City, a city of almost nine million people and notoriously heavy traffic, there is just one office, the headquarters of COFEPRIS, that can issue the stickers. Opening hours are 8am to 2pm, coinciding exactly with hours of medical consults in most hospitals. Dr. Argelia Lara, the head of the pain clinic at Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (hereinafter Hospital Nutrición), a tertiary level hospital in México City, told us:
By car it takes an hour to get to the office—if it isn’t rush hour, that is…another [hour] out there [at COFEPRIS] and another to return. In total three hours so you have to dedicate your whole morning to the process.[136]
Moreover, COFEPRIS in México City does not issue the barcoded stickers the same day. According to Dr. Lara, it usually takes two weeks before she can pick up the stickers. She notes that the waiting time varies greatly, making it difficult for her to plan. Dr. Lara uses a set of barcoded stickers every five weeks on average but the number of patients and their symptoms vary so it is important that she always has an ample supply of barcoded stickers. Otherwise, she may run out of them before having received a new set preventing her from prescribing opioid analgesics to patients who require them.
As Table 3 shows, some states informed Human Rights Watch that they charge a fee for either the license to prescribe opioids, packages of barcoded stickers or both, despite the fact that COFEPRIS maintains the process of obtaining barcoded stickers is free of charge.[137] It is unclear whether these charges are always enforced as some physicians told us that they were able to get the license or barcoded stickers free of charge even though the state government claims it charges a fee.
Numerous physicians interviewed during our research complained that the machines used to print barcoded stickers were antiquated and regularly broke down. As there was only one machine per state, this resulted in serious problems for physicians and their patients when the machine was out of order. In México City, for example, the machine broke down in 2012 and was out of order for a period of five months. Dr. Lara of Hospital Nutrición told Human Rights Watch:
We did not have prescriptions for almost five months and they did nothing. [For a long time] there was no resolution and nobody was providing anyone any information on what was happening.
She said that the situation has improved somewhat since that time: “Before it was horrible. The environment was chaotic. The system went down all the time. Now it is a bit better and the place is larger and has more personnel.”[138]
The machine also broke down for an extended period of time in Veracruz in 2013. Dr. Rafael Contreras of the Centro Estatal de Cancerología del Estado de Veracruz told Human Rights Watch in March 2013 that because of the malfunction, barcoded stickers for Veracruz were being printed in Puebla, a neighboring state. As a result, it took more than a month from the time new stickers were requested until they could be picked up.[139]
Under the announced reforms to the prescribing system for this class of medicines, the government plans to institute an electronic process for barcoded stickers. Physicians will be able to request 100 sets of barcoded stickers at a time, download these onto their computers and print them.[140] Provided the electronic system works smoothly, it should eliminate most of the difficulties physicians currently encounter in obtaining barcoded stickers.
Licensing of Pharmacies
Under Mexican regulations, pharmacies must obtain a special license to be able to buy and sell opioid medications and they must keep detailed books of any sales.[141] We interviewed about a dozen managers of pharmacies, who had obtained this license or were in the process of doing so, and they described the process as relatively unproblematic. They said obtaining the license did require a certain amount of paperwork and that it can take several months to receive final approval but that was not overly complex. They also said that record keeping requirements are more stringent than for regular medications, although they did not consider that to be a significant problem.[142]
In contrast, about a dozen managers and other staff of pharmacies who did not have the license often cited the perceived hassle of carrying opioid analgesics. They said that it would be difficult to get the license, that the paperwork involved in accounting for the comings and goings of these medicines would be cumbersome, and that they would be subject to additional and invasive inspections by officials of their management of opioid analgesics.
But the main reason these pharmacy staff identified for not getting the license was the lack of demand for these medicines. The manager of a pharmacy in Tepatitlán, Jalisco, for example, told us: “It doesn’t make economic sense. We can’t return these medicines so they will expire. People don’t request the medicines.”[143] Given how few physicians prescribe these medicines, especially outside of the major cities, this is hardly surprising.
Several pharmacists identified the fact that they must provide the state health department with a projection for the amount of opioid analgesics they expect to need for a six month period as a problem. They said that if it turns out during that period that they need more of a certain medication they have to wait for the next six month period to adjust their estimate.[144] As a result, pharmacies may run out of the medication, which can be difficult for patients as so few pharmacies carry opioid analgesics.
Problems with Dispensing of Opioid Analgesics
How often do patients have to return to get a new prescription? All the time! – Pharmacy manager in Guadalajara[145]
Easily 15% of my barcoded stickers are lost…Why is it so difficult? It's hard for the doctor, hard for the patient and hard for the pharmacy. It should be much simpler – Dr. Jesús Medina[146]
In interviews with patients, family members, physicians and pharmacists we heard dozens of reports of pharmacies refusing to fill prescriptions for opioid analgesics because of errors or non-compliance with official rules. Some physicians estimated that pharmacies cannot fill as many of 15 to 30 percent of the prescriptions they write for opioid analgesics.
These refusals are major inconveniences for people with life-limiting illnesses and their relatives. When a pharmacy cannot fill their prescription they have to return to their physicians for a new prescription. Such a trip—followed by a second trip to the pharmacy—can take many hours and may temporarily leave the patient without their medicines. For doctors, rejected prescriptions are a problem because they lead to a waste of barcoded stickers that, as described above, are hard to get.
We found that the rejection of these prescriptions is generally due to a combination of factors, including limited and unreliable stock of these medicines at pharmacies, excessive caution in handling this class of medicines at some pharmacies, inspections by departments of health that sometimes appear to encourage this, and finally, a lack of flexibility in the prescribing and dispensing rules that would allow for the problem to be resolved without an extra trip to the physician.
Most of the rejections of prescriptions we learned about were related to one of the following requirements:
- The requirement that the prescription covers a period of no more than thirty days;[147]
- Challenges with correcting minor, technical errors on prescription forms for opioid analgesics by pharmacies;[148]
- The requirement that prescriptions for opioid analgesics contain both the commercial and generic name of the medicine;[149]
- The lack of authority for pharmacies to substitute the medicine or formulation indicated in the prescription form, even after consultation with the prescribing physician, if they are out of stock of the requested medicine.[150]
In a meeting with Human Rights Watch in September 2014, Mikel Arriola, the head of COFEPRIS, agreed to make two key changes to the prescribing rules, which could help avoid many of the cases of canceled prescriptions we documented.[151] He also agreed that COFEPRIS would hold periodic meetings with the pharmaceutical industry, pharmacies and prescribers to try to identify potential challenges with the supply of morphine so as to avoid stock-outs.
Compliance with the 30-day Prescription Limit
…at times you have to invent things: the dose, the number of tablets so that they don’t return them [the prescriptions]. – General practitioner[152]
Mexican prescription rules for opioid analgesics limit the number of days a prescription for such medicines may cover to thirty days.[153] This limitation is not unreasonable. Indeed, most countries in the region impose a similar limit, although a few countries—Belize, Paraguay, St Lucia and Uruguay—leave it to the discretion of the prescriber.[154]
Yet, this limit creates a number of significant problems in México because pharmacies only dispense ready-made packages of medications. In other words, pharmacies do not count out the exact number of tablets or ampoules indicated on the prescription form but sell pre-packaged units.[155] Current practice is that if the amount of medication in a package exceeds the amount prescribed for the thirty days period, the pharmacy cannot dispense.
The amount of medications a patient needs over a 30 day period often does not coincide with the contents of available prepackaged boxes.[156] For example, Dr. Carlos García, a general practitioner in Ajijic, Jalisco, told us: “If the box comes with 100 tablets but the patient only needs 60 they won’t sell you the prescription.”[157] Physicians told Human Rights Watch that this forces them to revert to trickery to be able to prescribe the medications. One pain specialist, who requested anonymity because of potential legal implications, said: “I can't leave my patients without medications. So I fill out the prescription as if it would end in 30 days even though I know it will last him [longer]...[158]
Many doctors said they give patients two prescriptions: One (on the official prescription form with bar coded sticker) that has fictional daily doses of the medication adjusted to fulfill regulatory requirements and used only to get the medications from the pharmacy; the other (on a separate regular prescription form) with actual instructions to the patient on the doses and frequency with which the medications should be taken. One doctor gave the following example:
Sometimes patients require just a little more than 100 [tablets]…say 120 tablets per month. To give them 120 tablets I have to prescribe two boxes of 100 tablets and invent that they have to take more [six per day instead of four]. Otherwise, they won’t give [the medication] to them. Imagine, if I need 120 tablets that means I’ll have 80 left over. So we’re complying with the rule. The rule isn’t violated. But sometimes the rule is so inflexible that it obliges one to do things like this.[159]
A system that forces physicians to write prescriptions that do not correspond to the actual dosage prescribed for the patient is obviously problematic, especially as it can lead to confusion among patients and their families regarding the instructed dosage.
Table 4 - Oral opioid formulations[160]
|
Medication |
Brand name |
Formulation |
Tablets per package |
|
Hydromorphone |
Liberaxim |
2mg |
100 tablets |
|
Methadone
|
Rubidexol |
5mg |
100 tablets |
| 10mg | 100 tablets | ||
| Amidone | 40mg | 100 tablets | |
|
Morphine
|
Analfin |
10mg |
20 or 100 tablets |
|
15mg |
20 or 100 tablets |
||
|
30mg |
20 or 100 tablets |
||
|
Graten |
30mg |
20 or 100 tablets | |
|
Oxycodone (immediate release)
|
Endocodil |
5mg |
15, 30, or 100 tablets |
|
10mg |
15, 30, or 100 tablets |
||
|
20mg |
15, 30, or 100 tablets | ||
| 40mg | 15, 30, or 100 tablets | ||
|
Oxycodone (w paracetamol) |
Plexicodim |
5mg/325mg |
100 tablets |
|
Oxycodone (extended release)
|
Oxycontin |
10mg |
30 tablets |
| 20mg | 30 tablets | ||
| 40mg | 30 tablets |
Commercial and generic names. The rules for special prescription forms do not directly require that the physician indicate both the generic and commercial name of the opioid analgesic.[161] However, the rules for health supplies determine that for medicines not included in México’s catalogue of generic medicines—and none of the opioid analgesics are—it is obligatory that the commercial name of the medicine be indicated on the prescription form.[162] The rules establish furthermore that when the prescription contains the commercial name of the medicine the pharmacy can only dispense that denomination and “may only substitute it when expressly authorized by the prescriber.”[163]
As a result of these rules, pharmacies refuse to dispense opioid analgesics if the commercial name of the medicine is not indicated in the prescription. Similarly, they are not allowed to dispense alternatives of the same medicine with a different commercial name, even if they consult with the prescribing physician. For example, if a patient has a prescription for morphine tablets under the commercial name Graten (produced by PISA Pharmaceuticals) but the pharmacy has only Analfin (morphine tablets produced by Tecnofarma) it cannot dispense the morphine when the dosage and formulation are the same.
Miguel Márquez, the brother of Alejandra, said that morphine had recently run out in various pharmacies in Guadalajara.
Right now there isn’t any in Farmacias Guadalajara or in Benavides [another pharmacy], only in Farmacias Especializadas. They have to sell them. It's necessary. This is for a pain that is sharp. It's as I said sometimes the pain hits her it can be for four hours and she cries and screams.[164]
While these rules were clearly not intended to complicate access to opioid analgesics, both doctors and patients told us this is a significant issue for them. The problem is particularly acute because pharmacies maintain limited stock of opioid analgesics due to the limited use of these medicines in México and the fact that supply is not always reliable. As a result, pharmacies frequently run out of stock of certain medicines and/or formulations.
In the above-mentioned meeting with Human Rights Watch, COFEPRIS agreed to a change in prescription rules to allow physicians to indicate only the active substance (or generic name) on prescription forms as opposed to the commercial name.[165]
Lack of flexibility to make technical corrections to prescriptions for opioid analgesics. Under current regulations, only the person in charge of pharmacies, the responsable sanitario, can correct technical or clerical errors on the prescription after consulting the prescribing physician. When this official is not present, which is very common, México’s rules do not permit corrections so the patient must return to the physician for a new prescription. Errors may include an illegible diagnosis, a mistake in the spelling of the name of the patient, or an error in the formulation of the medication (for example, 50mg tablets of methadone, which do not exist in México, instead of 5mg). A doctor at a pain clinic in Guadalajara, for example, said:
There are pharmacies that are very over sensitive. If I put Sector Libertario [a neighborhood in Guadalajara] as “SL” or if I put down “GDL” or “GAL” [for Guadalajara], they refuse to fill it.[166]
The WHO recommends that “in order to start the prescribed therapy in a timely manner, legislation should address the pharmacist’s ability to correct technical errors in prescriptions.”[167]
Substituting formulation of medication. Under current rules, there is no mechanism to adjust the formulation of opioid analgesics when the formulation indicated on the prescription form is not available. Thus at present, a pharmacy would not be able to dispense 10mg morphine tablets to a patient with a prescription for 30mg morphine tablets even if the latter are unavailable. Patients told us that as stock is often limited and few pharmacies carry opioid analgesics, which is frequently a problem. At present, even a faxed or emailed correction from the prescribing physician is not sufficient to allow the pharmacy to substitute the formulation. Dr Jesús Medina of Instituto Palia told Human Rights Watch: “Patients often return. They say: ‘Doctor, this medication is out. They don't have it in the pharmacy. They only have this strength. What do I do?’ ‘Doctor, can you write a new prescription?’”[168]
During the above-mentioned meeting, the head of COFEPRIS suggested that prescription rules could be changed to allow physicians to indicate multiple alternatives for dosages on prescription forms.[169] In other words, physicians would be able to indicate that the pharmacist could dispense tablets of 30mg or twice the number of tablets of 15mg. Such change to the rules would greatly facilitate the dispensing of these medicines.
Some pharmacists told us that inspections by the health department had made them more cautious about dispensing opioid analgesics. For example, the manager at Farmacia Maypo in Guadalajara said the health department had criticized him for dispensing a prescription for one hundred tablets of morphine that did not specify whether it should consist of one box of one hundred tablets or five boxes of twenty. The manager said:
We did not have that kind of control before. Before, you could say: “Here you go” when you got that kind of prescription. Since they came, we [reject] prescriptions more frequently.[170]
The manager of another pharmacy told us: “There are complaints because of the formulations. They fine us. With controlled medicines, there is no flexibility. They are very strict.”[171]
Stock-Outs of Morphine
Medical doctors from each of the regions where we conducted research said they frequently faced stock-outs of opioid medicines. A palliative care doctor from Chiapas said, for example: “Stock-outs of morphine are a key factor that limits our use [of the medicine].”[172] To obtain a snapshot of current stock-outs, we surveyed palliative care physicians and pharmacists at institutions in Chiapas, Jalisco, Nuevo León and México City who told us the following about stock-outs they had experienced in 2014.
Jalisco: “At this moment we have a stock-out of morphine in tablets in the metropolitan area [of Guadalajara]. We don’t have tablets of 15 and 30mg of either brand. There are just a few boxes of Analfin [one of the brands] with 20 tablets of 10mg left. This has been going on for two weeks and according to the representative [of Tecnoforma, which produces Analfin] we won’t have [a new supply of morphine] until mid-September.”[173]
México City: “In April and May, we were altogether without morphine. Now there are tablets of 10mg but not of 15 or 30mg. I currently have a patient with advanced cancer for whom taking three times as many tablets is difficult because she has trouble swallowing. My only alternative is to prescribe Oxycodone, which is available, but six times more expensive.”[174]
Nuevo León: “We have not had difficulties with oral morphine in Monterrey but recently we’ve had a stock-out of injectable morphine. I currently have a patient with cancer who needs high doses of morphine and I have to switch her to different opioids because the pharmacy has been informed that they won’t get any more [stock in the immediate future].”[175]
Chiapas: “Pharmacies do not have stock of the different formulations or continuity of stock over time. This means we cannot maintain effective pain therapy over time.”[176]
We did not specifically investigate the reasons for these stock-outs although interviews suggest they are likely caused by a combination of factors, including lack of demand for these medicines, the limited number of pharmaceutical companies producing them, regulatory complexities around the importation of raw materials, inadequate communication, and problems with compliance with regulations on handling opioid materials. On September 12, 2014, COFEPRIS and México’s member of the International Narcotics Control Board, Dr. Alejandro Mohar-Betancourt, hosted a first meeting of pharmaceutical companies, pharmacies and healthcare providers to identify and overcome challenges with the supply of morphine.[177]
Barriers to Access to Opioid Analgesics in Home Care
While Mexican law encourages home-based palliative care current regulations do not authorize physicians who conduct home visits to patients to carry opioid analgesics.[178] Under current regulations home teams should leave families with prescriptions so that a family member can go to the pharmacy to buy the medications. This seems to defeat the purpose of home-based palliative care.
Some home teams have developed a trick that allows them to bring medications with them and leave them with the patients. As regulations allow people to carry opioid medications if they have a filled out prescription form with barcoded sticker, these home-care physicians write a fictitious prescription for opioid analgesics, with barcoded stickers, before they head out to visit patients in their homes. This prescription allows them to carry the medicines. At patients’ homes, they conduct an examination in order to determine the dosage the patient actually needs at which point they may need to write another prescription for the actual amount of medication the patient requires.
The existence of these restrictions is not surprising. Drug regulations in most countries were written before the home-based and primary-care models for palliative care began to develop. Regulations, however, need to be modernized to reflect current needs and practices. Just as Mexican regulations authorize—indeed oblige—certain ambulances to carry injectable morphine, they should specifically allow home-based palliative care teams to bring supplies of opioid medicines to their patients without having to waste prescription forms.[179]
Inclusion of Essential Palliative Care Medicines in México’s Medicines’ Lists
Since 2013, the WHO Model List of Essential Medicines contains a section on pain and palliative care that includes a total of twenty medications in three categories: non-opioids and non-steroidal anti-inflammatory medicines, opioid analgesics, and medicines for other common symptoms in palliative care patients. The WHO recommends that these medicines be available to all who need them in the formulations indicated in the list. The May 2014 World Health Assembly resolution recommends that all countries adjust their national medicines lists accordingly.
In México, the Council of Public Health (Consejo de Salubridad General) is responsible for preparing and updating the list of essential medicines (in Spanish: Cuadro Básico y Catálogo de Medicamentos). This list forms the basis of the medicines lists of México’s health insurers, such as Seguro Popular and IMSS.[180] The list was last updated in 2013.
A comparison of the WHO’s list and the medicines lists of the Council, Seguro Popular and IMSS reveal a number of important differences (see Appendix 2), including:[181]
- None of the Mexican lists contain a pain and palliative care section. Instead, as in earlier versions of the WHO’s list, palliative care medications are spread out over six different sections, including analgesia, anesthesia, psychiatry, rheumatology, gastroenterology and oncology. As a result, medicines that may be needed for the provision of palliative care to a patient with heart disease may be categorized as medicines for cancer or psychiatry. This may lead to reluctance among physicians to prescribe these medicines.
- Five medicines that the WHO considers essential for palliative care are not included in any of México’s lists: docusate sodium, cyclizine, hyoscine hydrobromide (on the WHO Model List of Essential Medicines for Children), ibuprofen, and lactulose. According to a Mexican pharmacist specialized in palliative care, México’s lists do not contain an adequate substitute for the first three of these medicines.[182]
- Key formulations, mostly oral solutions and suppositories, for eight medicines on the WHO list are missing from México’s lists.[183] This complicates administration to patients who have trouble swallowing or have uncontrollable nausea, neither of which is uncommon in palliative care patients. Oral solutions are also essential for many young children.
- Finally, México’s essential medicines lists specify at what levels of care medicines should be available whereas the WHO recommends that all essential medicines be available at all levels of care.[184] The Council’s list, for example, designates six of the essential palliative care medicines—opioid analgesics, several medicines routinely used in patients with anxiety, and an anti-nausea medicine—for use only in secondary and tertiary care facilities.
These discrepancies are likely to lead to inadequate treatment for patients who require palliative care.